Collaborative Funding Partners
CIRM funds research carried out in California. However, excellent stem cell science is taking place worldwide. That’s why CIRM supports partnering opportunities with researchers outside California and the US. CIRM 2.0 allows researchers from around the globe to bring their work to California where it can be funded.
Also, California scientists can propose to collaborate with their innovative colleagues around the globe. California scientists may propose to collaborate with partners who bring unique scientific credentials and/or funding to perform research in their home jurisdictions. Bringing additional capacities and resources can serve to strengthen scientific proposals and thus increase the likelihood of successful funding.
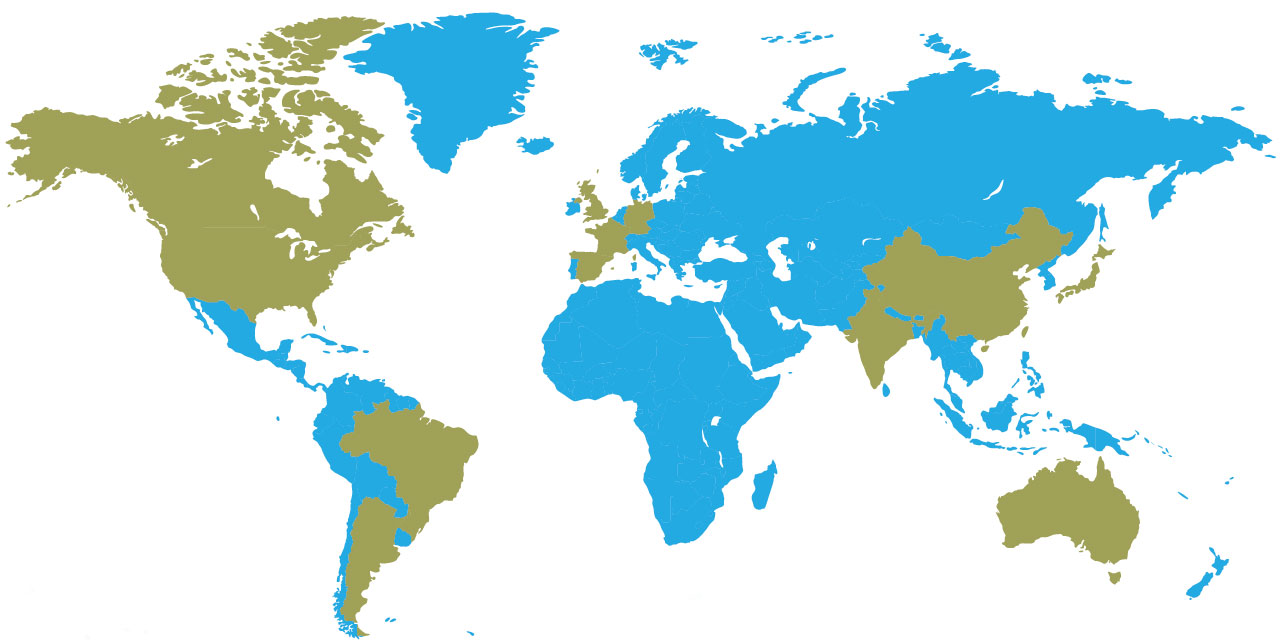
In the map above, countries in green indicate funding relationships with CIRM.
The list of Approved Funds (below) represents the current award amount under agreement with the grantee which can be less the amount approved by the governing board. For projects approved by the governing that have not yet been issued an award agreement, the Approved Funds will temporarily reflect the amount approved by the governing board.
