From Bench to Bedside
The development pipeline guides therapies from early-stage discovery through preclinical research and into clinical application.

Taking a therapy from an idea in a research lab to testing in clinical trials is no small feat, but an established regulatory pathway guides the process. CIRM accelerates the journey from lab breakthroughs to life-saving treatments by providing funding support at every stage.
The Development Pipeline
How does an idea become a medicine?
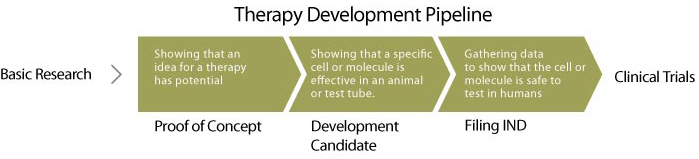
Early Research:
Discovery Research
In the discovery phase, ideas for cell and gene therapies are tested in research labs. For example, in prior basic research, scientists might have identified a way to induce stem cells to make neurons, and they hypothesize that these neurons could repair a spinal cord injury. The scientists would then test this therapeutic candidate (the neurons) in animal or other model systems of disease, to determine if the candidate has reproducible disease-modifying activity. Candidates that show these desirable effects are called development candidates and advance to translational development.
Mid-stage Research:
Translational Research
In the translational phase, scientists do more work to bolster the idea that the development candidate could feasibly be manufactured and administered as a therapy. They work to develop a reproducible and scalable manufacturing process and carry out preliminary safety experiments in animals. They may also experiment with optimizing the efficacious dose in the animal model, and the best way to administer the therapy. After gathering all these data, the scientists arrange a pre-investigational new drug (pre-IND) meeting with the Food and Drug Administration (FDA) during which they obtain FDA feedback on the remaining preclinical work they will do prior to applying for permission to carry out a clinical trial in humans.
Photo Credit: Cal Poly Humboldt
Late-stage Research:
IND-enabling research
After the pre-IND meeting, the investigators must manufacture the product using Good Manufacturing Practice (GMP) methods, as is required before testing the product in humans. In addition, they carry out formal Good Laboratory Practice (GLP) safety studies in animal models using the same dosing range and frequency that would be intended for use in humans, and they plan out a formal clinical protocol for a clinical trial. All this information is submitted to the FDA in the form of an investigational new drug application (IND). If the FDA is satisfied with all the information provided, they will give permission for the investigators (clinical trial sponsors) to initiate a clinical trial to test the safety and in humans.
Clinical Research:
The discovery, translational, and preclinical research all provide data to support the rationale to test the candidate in humans. Traditionally, clinical trials proceed in three phases, each of which must be reviewed by the FDA prior to advancing to the next stage.
Clinical trials include a very small number of people and are primarily intended to test whether the proposed therapy is safe, but also to look for early signs that it may be efficacious in treating the disease. In addition, different dose levels or dosing regimens may be explored.
Clinical trials include slightly more people and continue to monitor safety while starting to look more rigorously at signs that the therapeutic is efficacious.
Clinical trials are done if there are signs that the therapy has efficacy in the Phase 2 trial. These trials include larger numbers of people and usually have a control arm, in order to get statistical data supporting the idea that the therapy is truly benefiting patients.
Commercialization
After Phase 3, the sponsor may apply for a license to manufacture and sell the drug. For cell and gene therapies, this is called a Biological License Application (BLA). FDA reviews all the preclinical, manufacturing, and clinical trial data, looking for evidence that patients benefit from taking the therapy and that this benefit outweighs any safety risks. If the FDA decides the therapy is safe and effective, it may grant label approval. Then, and only then, the therapy can be used by doctors to treat patients.

