Cell and Gene Therapy Center
Cell and gene therapies face many headwinds: complex preclinical development, constrained GMP manufacturing capacity, limited qualified clinical trial sites, an evolving regulatory landscape and an unclear pathway for pricing and market access. To tackle these challenges, the California Institute for Regenerative Medicine (CIRM) has partnered with IQVIA to form the IQVIA Cell And Gene Therapy Center (CAGT Center, formerly Stem Cell Center). Since its founding in 2016, the CAGT Center has supported over 20 CIRM awardees in cell and gene therapy development from candidate identification to marketing authorization.
Established expertise:
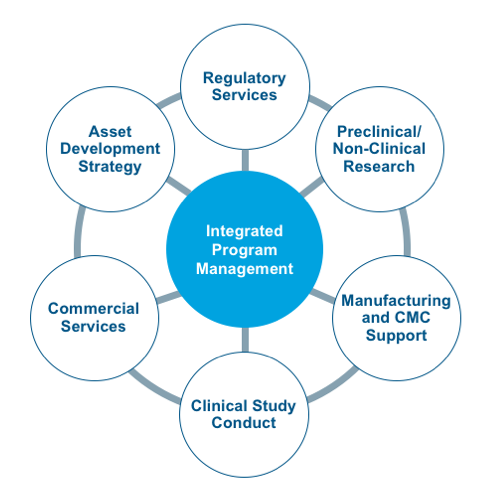
The CAGT Center includes a dedicated team of experts with an average of 15+ years of cell and gene therapy-specific expertise in:
- Product development through pre-clinical, clinical and market access
- CMC, process development, and GMP manufacturing
- Regulatory strategy and operations
- Clinical study execution
The CAGT Center leverages the capabilities of IQVIA, a leading global provider of big data/information, innovative technology solutions, and contract research services, to provide insights for every challenge along the value chain. IQVIA’s comprehensive network of scientific, therapeutic, operational, drug development, and commercialization experts provide expertise for any need.
Customized strategic and operational services:
The IQVIA Cell And Gene Therapy Center provides flexible and customized support for every need in the development pathway. The center has a proven track record supporting a range of CIRM awardees and other clients, from academics to small biotech to large pharmaceutical companies, in:
- Evaluating the specific needs of the cell and gene therapy programs to develop a strategic GMP manufacturing plan that reduces complexity, time, and risk
- Developing a regulatory strategy to facilitate successful INTERACT, pre-IND / IND meetings and achievement of expedited pathway designations (e.g., RMAT)
- Preparing a Target Product Profile to guide the development plan and communicate product attributes and potential value to stakeholders
- Executing clinical trials, including identifying sites with the infrastructure and experience required capabilities for advanced technologies and recruiting hard-to-find patients
- Preparing evidence for pricing and reimbursement to navigate market access and uptake barriers

IQVIA Cell and Gene Therapy Fact Sheet
IQVIA Cell And Gene Therapy Center Publications:
Making the Move: Bringing Cell and Gene Therapy Development Programs to the United States
Experienced network of partners:
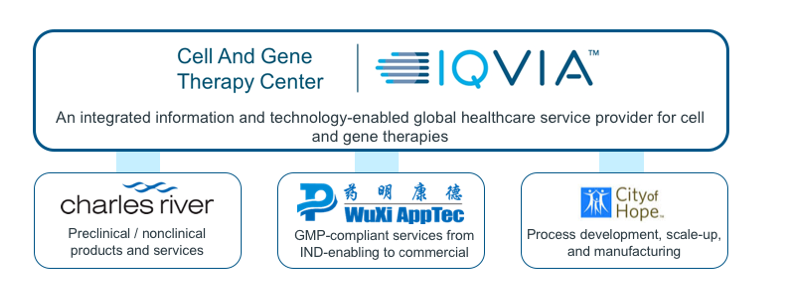
The CAGT Center works in close collaboration with a group of alliance partners to address the unique challenges in cell and gene therapy manufacturing and nonclinical research.
Want to learn more about the services the CAGT Center offers?
Please refer to the following document: IQVIA Cell And Gene Therapy (CAGT) Center: Core Services for CIRM Awardees (PDF)
Contacting the CAGT Center 1,2
To inquire about the CAGT Center services, please contact:
- Email: CAGT@iqvia.com
- Phone: +1 858-646-2108
- Address: 10188 Telesis Court Suite 400, San Diego, CA 92121
1 – If you are contacting the CAGT Center regarding CIRM translational awards or applications and would like CIRM input on eligibility, please include translational@cirm.ca.gov with your correspondence.
2 – If you are contacting the CAGT Center regarding CIRM clinical awards or applications and would like CIRM input, please include clinical@cirm.ca.gov with your correspondence.
