Active Awards Portfolio Dashboard

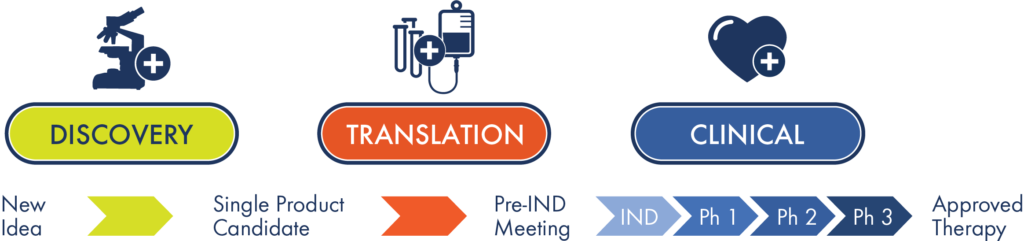
It takes a lot of effort to develop a promising stem cell research idea into an effective treatment that can help patients. CIRM funds a pipeline of projects spanning discovery, translation and clinical stage research. Check out our portfolio of active research awards in our interactive dashboard below. For a detailed list of CIRM-funded clinical trials, visit our Clinical Trials Dashboard.
| Disease | Cell Type | Therapeutic or Technology | Institution | Stage | Project Objective | Details |
|---|---|---|---|---|---|---|
| Autism, Neurological Disorders | iPS Cell | Bioinformatics/Computational Tools, Cell Line Generation-Resource, Exploring disease mechanisms, Model system development, Technology | UCSD |  |
Research Insights | |
| Developmental Disorders, Intellectual Disability, Neurological Disorders, Pediatrics | iPS Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Exploring therapeutic Mechanism, Technology | UCSF |  |
Research Insights | |
| Autism, Intellectual Disability, Neurological Disorders | iPS Cell | Exploring disease mechanisms, Technology, Therapeutic target discovery | UCLA |  |
Proof of Concept, Research Insights | |
| Autism, Neurological Disorders | iPS Cell | Bioinformatics/Computational Tools, Exploring disease mechanisms, Gene Therapy (All), Gene Therapy, cell free, Nucleic acid based therapy, Technology, Therapeutic Approach, Therapeutic target discovery | Scripps Research |  |
Research Insights | |
| Neurological Disorders, Other | iPS Cell | Cell Line Generation-Resource, Exploring disease mechanisms, Gene editing tools, Specific Cell Type Derivation, Technology | UCSF |  |
Research Insights, Tool/Resources/Bottleneck | |
| Autism, Neurological Disorders | iPS Cell | Exploring disease mechanisms, Model system development, Technology | UCSF |  |
Research Insights | |
| Autism, Intellectual Disability, Neurological Disorders | iPS Cell | Exploring disease mechanisms, Technology | Scripps Research |  |
Research Insights | |
| Heart Disease, Hypertension, pulmonary arterial, Pulmonary Hypertension | iPS Cell | Exploring disease mechanisms, Technology | Stanford |  |
Research Insights, Tool/Resources/Bottleneck | |
| Dementia, Neurological Disorders, Parkinson's Disease | iPS Cell | Exploring disease mechanisms, Technology | Gladstone |  |
Research Insights | |
| Duchenne Muscular Dystrophy, Muscular Dystrophy, Skeletal/Smooth Muscle disorders | Adult or Tissue Stem Cell | Exploring therapeutic Mechanism, Technology | UCLA |  |
Research Insights | |
| Infectious Disease, Neurological Disorders | iPS Cell | Bioinformatics/Computational Tools, Exploring disease mechanisms, Technology | UCSF |  |
Research Insights | |
| Diabetes, Metabolic Disorders, Type 1 diabetes | Embryonic Stem Cell, iPS Cell | Immune tolerance/management, Technology | Minutia, Inc. |  |
Research Insights | |
| Skeletal/Smooth Muscle disorders | Adult or Tissue Stem Cell | UCSD |  |
Research Insights | ||
| Blood Cancer, Cancer, Leukemia, Acute Myeloid (AML) | Cancer Stem Cell | Exploring disease mechanisms, Technology | Sanford-Burnham |  |
Research Insights | |
| Developmental Disorders, Genetic Disorder, Neurological Disorders, Pediatrics | iPS Cell | Cedars-Sinai |  |
Research Insights | ||
| Infectious Disease, Neurological Disorders, Zika virus | Embryonic Stem Cell, iPS Cell | Exploring disease mechanisms, Technology | UCLA |  |
Research Insights | |
| Aging | Adult or Tissue Stem Cell, iPS Cell | Exploring stem cell mechanisms, Specific Cell Type Derivation, Technology | COH |  |
Research Insights | |
| Developmental Disorders, Genetic Disorder, Respiratory Disorders | iPS Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Model system development, Technology | Lundquist Institute |  |
Research Insights | |
| Developmental Disorders, Genetic Disorder, Intellectual Disability, Neurological Disorders, Rett's Syndrome | iPS Cell | Exploring disease mechanisms, Technology | UCLA |  |
Research Insights | |
| Intestinal Disease, Metabolic Disorders, Neurological Disorders, Other | iPS Cell | Personalized cell therapy, Therapeutic Approach | UCSF |  |
Proof of Concept, Research Insights | |
| Neurological Disorders | Embryonic Stem Cell | Bioinformatics/Computational Tools, Exploring stem cell mechanisms, Model system development, Technology, Tissue engineering | UCSC |  |
Research Insights, Tool/Resources/Bottleneck | |
| Lung Disease, Fibrosis, Respiratory Disorders | Adult or Tissue Stem Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Technology, Therapeutic target discovery | UCSF |  |
Research Insights | |
| Fertility | Embryonic Stem Cell, iPS Cell | Model system development, Technology | UCLA |  |
Research Insights, Tool/Resources/Bottleneck | |
| Retinitis Pigmentosa, Vision Loss | iPS Cell | Exploring disease mechanisms, Technology | UCSF |  |
Research Insights, Tool/Resources/Bottleneck | |
| Cystic Fibrosis, Respiratory Disorders | Adult or Tissue Stem Cell | Cell delivery or targeting, Gene editing tools, Technology | UCLA |  |
Research Insights, Tool/Resources/Bottleneck | |
| Amyotrophic Lateral Sclerosis, Neurological Disorders | iPS Cell | Gene Therapy (All), Gene Therapy, cell free, Nucleic acid based therapy, Therapeutic Approach | UC Irvine |  |
Proof of Concept, Research Insights | |
| Neurological Disorders, Traumatic Brain Injury | Embryonic Stem Cell | UC Irvine |  |
Research Insights | ||
| Neurological Disorders | Adult or Tissue Stem Cell, iPS Cell | Exploring stem cell mechanisms, Specific Cell Type Derivation, Technology | UCSF |  |
Research Insights | |
| Autism, Developmental Disorders, Neurological Disorders | iPS Cell | Bioinformatics/Computational Tools, Exploring disease mechanisms, Exploring stem cell mechanisms, Technology | Scripps Research |  |
Research Insights | |
| Cancer | iPS Cell | Gene Therapy (All), Gene-modified, personalized cell therapy, Immune tolerance/management, Specific Cell Type Derivation, Technology, Therapeutic Approach | UCLA |  |
Research Insights, Tool/Resources/Bottleneck | |
| iPS Cell | Assay development, Bioinformatics/Computational Tools, Exploring stem cell mechanisms, Technology | UCSD |  |
Research Insights, Tool/Resources/Bottleneck | ||
| Brain Injury, hypoxic, ischemic, Neurological Disorders, Pediatrics, Vascular Disease | Adult or Tissue Stem Cell | Exploring stem cell mechanisms, Technology | UCSF |  |
Research Insights | |
| Blood Cancer, Blood Disorders, Cancer | Adult or Tissue Stem Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Technology | UCSC |  |
Research Insights | |
| Neurological Disorders, Neuropathy, Toxicity | iPS Cell | Cell imaging & tracking, Exploring disease mechanisms, Specific Cell Type Derivation, Technology, Therapeutic target discovery | UCSF |  |
Research Insights | |
| Amyotrophic Lateral Sclerosis, Neurological Disorders | iPS Cell | Model system development, Technology | Gladstone |  |
Research Insights | |
| Bone or Cartilage Disease, Tendon/Ligament/Connective Tissue Injury or Disorder | iPS Cell | Donor cell therapy, Therapeutic Approach | Cedars-Sinai |  |
Proof of Concept, Research Insights | |
| Heart Disease | iPS Cell | Small molecule therapy, Therapeutic Approach | Greenstone Biosciences |  |
Research Insights, Tool/Resources/Bottleneck | |
| Fertility | Embryonic Stem Cell, Other | Model system development, Technology | UCSD |  |
Research Insights, Tool/Resources/Bottleneck | |
| Neurological Disorders | iPS Cell | Cell delivery or targeting, Technology | Stanford |  |
Research Insights | |
| Neurological Disorders, Rett's Syndrome | iPS Cell | Gene editing tools, Model system development, Technology | UCSD |  |
Research Insights, Tool/Resources/Bottleneck | |
| Cancer, Solid Tumors | iPS Cell | Gene Therapy (All), Gene-modified, donor cell therapy, Therapeutic Approach | UCSF |  |
Proof of Concept, Research Insights | |
| Huntington's Disease, Neurological Disorders | Embryonic Stem Cell, iPS Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Technology | UC Berkeley |  |
Research Insights | |
| Lung Disease, Fibrosis, Respiratory Disorders | Adult or Tissue Stem Cell | Exploring disease mechanisms, Exploring stem cell mechanisms, Technology | UCSF |  |
Research Insights | |
| Muscular Dystrophy, Skeletal/Smooth Muscle disorders | iPS Cell | Cell delivery or targeting, Gene editing tools, Technology | UCLA |  |
Tool/Resources/Bottleneck | |
| Fertility, Genetic Disorder | Adult or Tissue Stem Cell | Exploring stem cell mechanisms, Technology | UCSD |  |
Research Insights, Tool/Resources/Bottleneck | |
| Age-related macular degeneration, Retinitis Pigmentosa, Vision Loss | Directly Reprogrammed Cell | Donor cell therapy, Specific Cell Type Derivation, Technology, Therapeutic Approach | CHLA |  |
Development Candidate, Proof of Concept | |
| Brain Cancer, Cancer, Solid Tumors | Cancer Stem Cell | Biologic, Gene modification, Therapeutic Approach | UC Irvine |  |
Development Candidate, Proof of Concept | |
| Amyotrophic Lateral Sclerosis, Neurological Disorders | iPS Cell | Gene Therapy (All), Gene Therapy, cell free, Therapeutic Approach | UCSF |  |
Development Candidate | |
| HIV/AIDS, Infectious Disease | Gene Therapy (All), Gene Therapy, cell free, Therapeutic Approach | UCLA |  |
Development Candidate | ||
| Heart Disease, Heart failure | Embryonic Stem Cell, iPS Cell | Gene Therapy (All), Gene Therapy, cell free, Therapeutic Approach | UCSD |  |
Development Candidate |



