
For immediate release
Contact:
Koren Temple-Perry
Sr. Director, Marketing & Communications
510-775-2314
press@cirm.ca.gov
South San Francisco, CA – The California Institute for Regenerative Medicine (CIRM), the world’s largest institution dedicated to regenerative medicine, has awarded $17.5 million to fund clinical-stage research projects aimed at advancing stem cell and gene therapy treatments for a variety of conditions ranging from neurodegenerative diseases to blood cancers.
The awards will support three projects in the Agency’s clinical program which provides funding for eligible stem cell and gene therapy-based projects through all stages of clinical trial development. These awards bring the number of CIRM-funded clinical trials to 96.
The awards include:
| Application | Program Title | Principal Investigator / Institution | Amount |
| CLIN1-14945 | Pre-Clinical to Clinical Gene Therapy Development for CMT4J | Pirovolakis, Terry – Elpida Therapeutics | $3,930,964 |
| CLIN1-15060 | A 1XX-enhanced and fully non-viral BCMA chimeric antigen receptor (CAR) T cell therapy for Relapsed and Refractory Multiple Myeloma | Martin, Thomas – UCSF | $4,585,501 |
| CLIN2-15115 | The CuRe Trial: Cellular Therapy for In Utero Repair of Myelomeningocele | Farmer, Diana L. – UC Davis | $8,996,477 |
This month’s clinical awards include two preclinical projects and one clinical-stage project. Among the awards is a $9 million grant to Diana Farmer, MD at UC Davis and chief of pediatric surgery at Shriners Children’s Northern California to support the completion of a Phase 2 clinical trial to improve the outcomes for children born with a severe form of spina bifida called myelomeningocele (MMC).
MMC is a birth defect that occurs when the spine and spinal cord aren’t formed properly during fetal development, usually resulting in life-long lower body paralysis and bowel and bladder dysfunction.
The CIRM award will support the advancement of a one-of-a-kind treatment delivered while a fetus is still developing in the mother’s womb via a patch coated with placenta derived stem cells. The treatment is meant to prevent paralysis and preserve normal bodily function, and ultimately improve quality of life of these affected children and their families. Learn more about the procedure here.
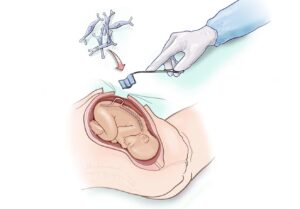
Image credit: UC Davis Health
“This award supplies critical funding to support a Phase 2 clinical trial to achieve our goal of using stem cells before birth to improve the ability to walk and have bowel and bladder control in patients born with spina bifida,” said Farmer, distinguished professor of surgery at UC Davis, chief of pediatric surgery at Shriners Children’s Northern California and principal investigator for the project, also known as the CuRe Trial. UC Davis Children’s Hospital and Shriners Children’s Northern California have one of the largest and most well-known spina bifida programs in the nation.
CIRM has funded several stages of Farmer’s research, including preclinical research and the completion of a Phase 1 clinical safety study which indicated no safety concerns in six patients.
“We are very grateful and proud to have CIRM and Shriners Children’s as partners for Phase 2 of the CuRe trial,” said Aijun Wang, PhD, co-principal investigator with Farmer on the project. Shriners Children’s is co-funding the trial.
“It is this important partnership that is making this groundbreaking work possible. This is also a testimony to the team science approach that our team has always been taking to conduct innovative research and solve unmet medical needs.”
In addition to the clinical-stage project, CIRM is supporting two preclinical stage projects. These include a $4 million grant to advance a gene therapy for Charcot-Marie-Tooth disease type 4J (CMT4J), a rare, debilitating, progressive hereditary motor and sensory neurological disease. Also included is a $4.6 million grant to produce a CAR-T cell therapy for multiple myeloma, the second most common malignancy among blood cancers.
For more information on CIRM’s clinical stage program, please visit CIRM’s funding opportunities page.
About the California Institute for Regenerative Medicine (CIRM)
At CIRM, we never forget that we were created by the people of California to accelerate stem cell treatments to patients with unmet medical needs, and act with a sense of urgency to succeed in that mission.
To meet this challenge, our team of highly trained and experienced professionals actively partners with both academia and industry in a hands-on, entrepreneurial environment to fast track the development of today’s most promising stem cell technologies.
With $5.5 billion in funding and more than 150 active stem cell programs in our portfolio, CIRM is one of the world’s largest institutions dedicated to helping people by bringing the future of cellular medicine closer to reality.
For more information go to www.cirm.ca.gov



