Osteoporosis, Bone and Cartilage Disease Fact Sheet
CIRM funds many projects seeking to better understand bone related diseases including osteoporosis, osteoarthritis and osteonecrosis to translate those discoveries into new therapies.
Description
Estimates of the number of people in the U.S. with osteoporosis range from 10 million to 25 million, with 75 percent being women. For many of those individuals it can be a disease with minimal immediate impact but incredible lingering risk. Between 1.5 million and 2 million of those with the condition develop osteoporosis-related fractures each year. About 70 percent of fractures are in the vertebra of the spine, and they can range from minor to completely debilitating. The next most common fracture is in the hip, which increases the risk of premature death and frequently lands otherwise healthy functional elderly in nursing homes for the remainder of their lives. Osteoporosis costs the nation an estimated $19 billion a year.
Osteoarthritis is a disease that affects the cartilage in joints. It is one of the most common forms of disability, effecting more than 27 million people in the U.S. CIRM funds several projects looking to replace or repair the cartilage lost in the degenerative disease. Projects include creating new cartilage from donor stem cells as well as developing a drug to drive a person’s own stem cells to do a better job of repair.
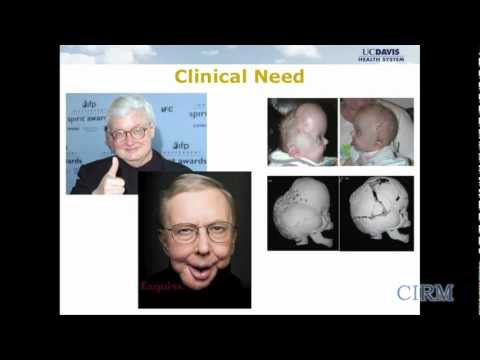
Osteonecrosis is a disease that decreases blood circulation to bones causing them to weaken and eventually die. If left untreated, patients with osteonecrosis can develop end-stage hip arthritis and require surgical joint replacement. Despite the low prevalence compared to primary osteoarthritis or degenerative arthritis, femoral head (hip) osteonecrosis has a significant economic impact because it largely affects individuals in the prime of life (peak age 35 years).
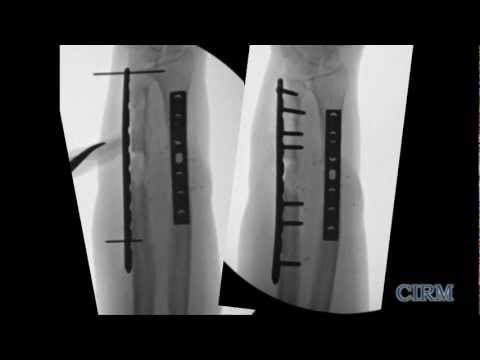
CIRM also funds project to help the thousands who live with fractures too big to heal or fractures in older individuals that their aging bodies can no longer heal. Between the ages of 30 and 80 we have a 10-fold decrease in the number of these stem cells and the ones we have left are less effective at replacing and repairing bone. California’s stem cell agency has funded several projects that propose various ways to increase the number of these stem cells, or improve their effectiveness to help keep bones healthier longer (the full list of CIRM awards is below).
Clinical Stage Programs
California Institute for Biomedical Research
Researchers at the California Institute for Biomedical Research (CALIBR) have been awarded $8.447 million to test KA34, a drug that, in preclinical tests, recruits stem cells to create new cartilage in areas damaged by osteoarthritis. CIRM funded the research that developed this technology and now this Phase 1 trial will test this stem cell directed treatment in people with osteoarthritis of the knee, hopefully slowing down or even halting the progression of the disease.
UC Davis
A team at UC Davis has developed a new stem cell-based treatment for osteonecrosis. They are using a small molecule drug that targets the mesenchymal stem cells in a patient’s bone marrow and directs the stem cells to the surface of the bone where they then develop new bone tissue. The team is testing the safety and efficacy of this drug treatment in a Phase 1 clinical trial.
CIRM Grants Targeting Bone & Cartilage Disease
| Researcher Name | Institution | Grant Title | Grant Type | Award Amount |
| Dmitriy Sheyn | Cedars-Sinai Medical Center | The role of WNT and BMP signaling pathways in iPSC to iTenocyte step-wise differentiation for tendon repair | Foundation – Discovery Stage Research Projects | $1,516,563 |
| Dr. Darryl D. D’Lima | Scripps Health | Pluripotent Stem Cells for Tendon Tissue Engineering | Quest – Discovery Stage Research Projects | $2,734,163 |
| Dmitriy Sheyn | Cedars-Sinai Medical Center | Microgel encapsulated iPSC-derived notochordal cells to treat intervertebral disc degeneration and low back pain | Quest – Discovery Stage Research Projects | $2,020,166 |
| Dr. Darryl D. D’Lima | Scripps Health | Meniscal Repair and Regeneration | Quest – Discovery Stage Research Projects | $1,361,775 |
| Dr. Kevin Stone | The Stone Research Foundation for Sports Medicine and Arthritis | A Novel Therapy for Articular Cartilage Autologous Cellular Repair by Paste Grafting | Quest – Discovery Stage Research Projects | $1,180,309 |
| Alireza Moshaverinia | University of California, Los Angeles | Gingival mesenchymal stem cells as a novel treatment modality for periodontal tissue regeneration | Inception – Discovery Stage Research Projects | $194,483 |
| Dmitriy Sheyn | Cedars-Sinai Medical Center | IVD rejuvenation using iPSC-derived notochordal cells | Inception – Discovery Stage Research Projects | $241,992 |
| Dr. Ahmed Gamal Ibrahim | Cedars-Sinai Medical Center | Noncoding RNA drug TY1 as a therapeutic candidate for scleroderma and systemic sclerosis | Therapeutic Translational Research Projects | $2,590,224 |
| Dr. Farhad Parhami Phd, MBA | MAX BioPharma, Inc. | Therapeutic development of Oxy200, an oxysterol with bone anabolic and anti-resorptive properties for intervention in osteoporosis | Therapeutic Translational Research Projects | $1,400,000 |
| Dr. Denis A Evseenko Dr. | University of Southern California | Pluripotent stem cell-derived chondrocytes for articular cartilage repair | Therapeutic Translational Research Projects | $2,503,104 |
| Ying Zhu | Ankasa Regenerative Therapeutics, Inc. | An autologous somatic stem cell therapy for the treatment of osteonecrosis | Therapeutic Translational Research Projects | $2,088,780 |
| Professor Jeffrey Charles Lotz | University of California, San Francisco | 2023 Center for Dental, Oral, & Craniofacial Tissue & Organ Regeneration (C-DOCTOR) Annual Spring Conference | Conference II | $30,250 |
| Dr Robert Hayes | Immusoft Corporation | A Phase I Open Label Study to Evaluate the Safety and Tolerability of ISP-001 in Patients with Mucopolysaccharidosis Type 1 | Clinical Trial Stage Projects | $8,000,000 |
| Dr. Thomas W Chalberg Jr. | Genascence Corporation | A Phase 1b, Randomized, Blinded, Placebo-Controlled Dose-Ranging Study of GNSC-001 Evaluating Safety, Pharmacodynamics, and Biomarkers in Knee OA | Clinical Trial Stage Projects | $11,637,194 |
| Dr. Gayatri Rao | Rocket Pharmaceuticals, Inc. | A Phase I Clinical Trial for a Lentiviral Gene Therapy Targeting the TCIRG1 Gene for Infantile Malignant Osteopetrosis (IMO) | Clinical Trial Stage Projects | $1,010,000 |
| Dr. Kristen A Johnson | Calibr | Evaluation of the Safety and Tolerability of KA34 in a Phase 1, Double-Blind, Dose Escalation Trial in Patients with Knee Osteoarthritis | Clinical Trial Stage Projects | $8,447,523 |
| Kyriacos A Athanasiou | University of California, Irvine | Treatment of the TMJ disc complex | Late Stage Preclinical Projects | $6,000,000 |
| Frank Petrigliano | University of Southern California | Plurocart: a novel stem cell-based implant for articular cartilage restoration | Late Stage Preclinical Projects | $5,999,782 |
| Ying Zhu | Ankasa Regenerative Therapeutics, Inc. | IND-enabling development of ART352-L, an endogenous stem cell reactivation therapy to enhance bone healing in the elderly | Late Stage Preclinical Projects | $3,987,693 |
| Jack J. Wang | Cellular Biomedicine Group, Inc. | Allogenic human adipose-derived mesenchymal stem cells for the treatment of knee osteoarthritis | Late Stage Preclinical Projects | $1,200,000 |
| Peter G Schultz | Calibr | Development of a Chondrogenic Drug Candidate Targeting Resident Mesenchymal Stem Cells for the Treatment of Osteoarthritis | Late Stage Preclinical Projects | $1,667,832 |
| Peter G Schultz | Calibr | Development of a Chondrogenic Drug Candidate Targeting Cartilage-residing Mesenchymal Stem Cells for the Treatment of Osteoarthritis | Preclinical Development Awards | $2,306,703 |
| Dr. Darryl D. D’Lima | Scripps Health | Embryonic Stem Cell-Derived Chondroprogenitor Cells to Repair Osteochondral Defects | Preclinical Development Awards | $7,660,211 |
| Dr. J. Kent Leach | University of California, Davis | Multi-modal technology for non-destructive characterization of bioengineered tissues | Tools and Technologies III | $1,574,151 |
| Dr. Fan Yang PhD | Stanford University | Injectable Macroporous Matrices to Enhance Stem Cell Engraftment and Survival | Tools and Technologies III | $1,434,235 |
| Dr. Dan Gazit | Cedars-Sinai Medical Center | Gene Targeting to Endogenous Stem Cells for Segmental Bone Fracture Healing | Early Translational IV | $5,121,514 |
| Nancy E. Lane | University of California, Davis | Treatment of non-traumatic osteonecrosis with endogenous Mesenchymal stem cells | Disease Team Therapy Development – Research | $18,227,898 |
| Prof. Kyriacos A. Athanasiou Ph.D | University of California, Davis | Tissue engineered cartilage from autologous, dermis-isolated, adult, stem (DIAS) cells | Early Translational III | $1,735,703 |
| Dr. Dan Gazit | Cedars-Sinai Medical Center | Genetically Engineered Mesenchymal Stem Cells for the Treatment of Vertebral Compression Fractures. | Disease Team Therapy Planning I | $107,622 |
| Nancy E. Lane | University of California, Davis | Increasing the endogenous mesenchymal stem cells to the bone surface to treat osteoporosis | Disease Team Therapy Planning I | $107,750 |
| John Hood | Samumed, LLC | Clinical Development of an osteoinductive therapy to prevent osteoporosis-related fractures | Disease Team Therapy Planning I | $99,110 |
| Dr. Dan Gazit | Cedars-Sinai Medical Center | Systemic Adult Stem Cell Therapy for Osteoporosis-Related Vertebral Compression Fractures | Early Translational II | $1,927,698 |
| Prof. Bruno Peault | University of California, Los Angeles | Harnessing native fat-residing stem cells for bone regeneration | Early Translational II | $5,359,076 |
| Peter G. Schultz | Scripps Research Institute | Cartilage Regeneration by the Chondrogenic Small Molecule PRO1 during Osteoarthritis | Early Translational II | $6,047,249 |
| Dr. Songtao Shi | University of Southern California | Oral and Craniofacial Reconstruction Using Mesenchymal Stem Cells | New Faculty I | $3,242,651 |
| Dr. Jill Helms | Stanford University | Enhancing healing via Wnt-protein mediated activation of endogenous stem cells | Early Translational I | $6,464,126 |
| Dr. Darryl D. D’Lima | Scripps Health | Stem Cell-Based Therapy for Cartilage Regeneration and Osteoarthritis | Early Translational I | $3,118,431 |
| Dr. Gage Crump | University of Southern California | Skeletogenic Neural Crest Cells in Embryonic Development and Adult Regeneration of the Jaw | New Faculty II | $2,247,403 |
| Professor A. Hari Reddi | University of California, Davis | hESCs for Articular Cartilage Regeneration | SEED Grant | $301,703 |
| | | | Total:
$132,895,067.31 |
CIRM Bone & Cartilage Disease Videos
Resources
Return to Disease Fact Sheets
Find Out More:
Stem Cell FAQ | Stem Cell Videos | What We Fund