Heart Disease Fact Sheet
CIRM funds many projects seeking to better understand heart disease and to translate those discoveries into new therapies.
Description
Heart disease strikes in many forms, but collectively it causes one third of all deaths in the U.S. Many forms of heart disease have a common result—cardiomyopathy. While this is commonly called congestive heart failure (CHF), it is really just the heart becoming less efficient due to any number of causes, but the most common is loss of functioning heart muscle due to the damage caused by a heart attack. An estimated 4.8 million Americans have CHF, with 400,000 new cases diagnosed each year. Half die within five years.
Numerous clinical trials are underway testing a type of stem cell found in bone marrow, called mesenchymal stem cells or MSCs, to see if they are effective in treating the form of CHF that follows a heart attack. While those trials have shown some small improvements in patients the researchers have not found that the MSCs are creating replacement heart muscle. They think the improvements may be due to the MSCs creating new blood vessels that then help make the existing heart muscle healthier, or in other ways strengthening the existing tissue.
California’s stem cell agency has numerous awards looking into heart disease (the full list is below). Most of these involve looking for ways to create stem cells that can replace the damaged heart muscle, restoring the heart’s ability to efficiently pump blood around the body. Some researchers are looking to go beyond transplanting cells into the heart and are instead exploring the use of tissue engineering technologies, such as building artificial scaffolds in the lab and loading them with stem cells that, when placed in the heart, may stimulate the recovery of the muscle.
Other CIRM-funded researchers are working in the laboratory, looking at stem cells from heart disease patients to better understand the disease and even using those models to discover and test new drugs to see if they are effective in treating heart disease. Other researchers are trying to make a type of specialized heart cell called a pacemaker cell, which helps keep a proper rhythm to the heart’s beat.
We also fund projects that are trying to take promising therapies out of the laboratory and closer to being tested in people. In some cases, these awards also fund the early phase clinical trials to show that they are safe to use and, in some cases, show some signs of being effective.
Clinical Stage Programs
Cedars-Sinai Medical Center
Pulmonary arterial hypertension (PAH) is a progressive condition with no cure. Scientists at Cedars-Sinai Medical Center are using donor cells derived from the heart to reduce two hallmark symptoms of pulmonary hypertension: inflammation and high blood pressure in the blood vessels within the lungs. These conditions make the heart struggle to pump blood to the heart and lungs, and over time, can ultimately lead to heart failure. The aim of this treatment is to delay the progression of the disease.
Capricor (Heart failure and Duchenne Muscular Dystrophy-related heart failure)
Capricor is using donor cells derived from heart stem cells developed by Cedars-Sinai to treat patients developing heart failure after a heart attack. In early studies the cells appear to reduce scar tissue, promote blood vessel growth and improve heart function.
In a second trial, Capricor is using the same donor cells derived from heart stem cells to treat patients developing heart failure due to Duchenne Muscular Dystrophy. In early studies the cells appear to reduce scar tissue, promote blood vessel growth and improve heart function.
Cedars-Sinai Medical Center (Cardiomyopathy)
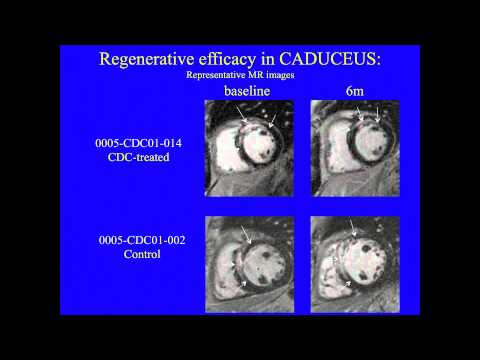
This team developed a way to isolate some heart-specific stem cells that are found in adult heart muscle. They use clumps of cells called Cardiospheres to reduce scarring caused by heart attacks. Initially they used cells obtained from the patient’s own heart but they later developed methods to obtain the cells they need from donor organs, which allows the procedure to become an off-the-shelf-therapy, meaning it can be available when and where the patient needs it rather than having to create it new each time. The company, working with the Cedars-Sinai team, received FDA approval to begin a clinical trial in June 2012.
Stanford School of Medicine (Heart Failure)
This team plans to turn embryonic stem cells into what are called cardiomyocytes, the kind of cells that can become heart muscle. They plan to develop methods for producing sufficient quantities for clinical therapy and to do all the laboratory work and preliminary testing needed to gain FDA approval of a clinical trial by the close of the grant. They are proposing to carry out a trial with patients who have disease that is so advanced that they are on a waiting list for heart transplants.
Video: Bruce Conklin of the Gladstone Institute of Cardiovascular Disease talks about using stem cells to screen drugs for heart side effects
CIRM Grants Targeting Heart Disease
CIRM Heart Disease Videos
Resources
- Blogs on heart disease research from the CIRM Stem Cellar
- NIH: Heart Failure Information
- Find a clinical trial near you: NIH Clinical Trials database
- American Heart Association
- National Heart, Lung and Blood Institute
- CDC: Heart Disease
Find Out More:
Stem Cell FAQ | Stem Cell Videos | What We Fund